By Fabio D'Agostino
The golden opportunity of a blockbuster (drug selling more
than $1bn per year) might have to be revisited for many Big Pharma and SMEs
which invested in cell and gene therapy development. Given the pressure due to
patents expiring and biosimilar competition, this should sound as a massive
relief. This is probably due to the fact that these products, arguably more
than other drugs, were born in University Research centres and therefore the
focus has been on development. Bio-preservation has been regarded as a trivial afterthought.
Not many developers seem to pay much attention to stability studies which are
paramount in order to design and develop the best supply chain option for the
product. As the industry moves from scientific curiosity to sustainable
commercialization, we know that preserving viability and potency of cell based
products is anything but simple. If for vaccines and monoclonal antibodies,
store and transport at 2-8 ⁰C is enough to preserve the product for 18 months
or even more, it is not that simple for cell based products. Bio-preservation
strategies will depend on cell type, temperature, excipients, primary container
(materials and shape). There are mainly 3 bio-preservation methods for cell
therapy medicinal products:
-
Controlled
ambient temperature: 15-25 ⁰C,
limited shelf life
-
Hypothermic
storage : 2-8 ⁰C, prolonged shelf life but normally requires addition of
protective agents ( like HypoThermosol, BioLife Solutions)
-
Cryopreservation:
- 80 ⁰C or below. It requires addition of toxic cryoprotectants (like DMSO)
which protects cells from ice crystals forming during cooling.
Because it normally enables the longest shelf life for the
cell-therapy products, cryopreservation is often regarded as the gold standard
or rather the “hoped” standard. Although it potentially equates to a more
stable product, the costs associated with dry nitrogen shipping and storage may
encourage developers to look at -80°C or even -20°C (should stability data
allow these temperatures). Cheaper storage and shipping fees will lower cost of
goods.
Another influencing factor for the storage conditions of the product
could also be the nature of the illness being treated. Acute illnesses being
treated by autologous therapies may not require long term storage of product if
the therapy is genuinely curative in nature. Furthermore, not every cell
therapy product can be effectively cryopreserved (with currently available
technology and methods).
World leading research groups have proved and disproved
the potential negative effect of cryopreservation on the therapeutic effect of
cell-based medicinal products. Many readers will agree there are so many variables
to take into consideration that perhaps nobody will ever be able to confidently
state that cryopreservation is safe and effective to use for cell-based
products. It might affect a particular mechanism of action while being
completely innocuous for others. Therefore, the key concern for developers
should be to validate a relevant potency assay and get the stability data
necessary to design the best bio-preservation option on a case by case basis.
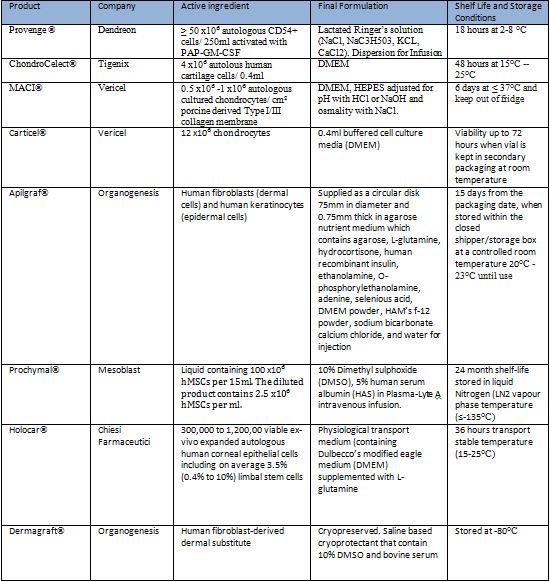
Here is a list of marked cell-based products, how many of
them are cryopreserved?
However, preserving viability and potency is only the first
step. For both autologous and allogeneic products, there are three key points
to consider:
§
Safety:
Full traceability of the entire supply chain, from donor to patient.
§
Scalability:
Operations should be designed in a way to manage an increasing number of
patients, from 1 per week in phase I to 10 or more in Phase III and commercial
stage. If Novartis hopes to launch its CTL019 in 2017, this might translate in
as many as 200 patients per week.
§
Flexibility:
This is important for both the company and the patient. The former needs to
coordinate with the hospital when to ship the product and the latter, who might
often been seriously ill, has too travel to the hospital on a set day.
It is the first time in history that big pharma company or
SMEs have to design a more service-based model than a product-based. For a physician and for the patient, what
will ultimately matter is that the drug does the trick, especially for life
threatening diseases. As we move towards
a more individualized medicine, one might envision that pharmaceutical
companies will make more profit from the service they provide than from their
products. Many industries have already witnessed such paradigm shift. Companies like TrakCel are leading the
way in proving cell and gene therapy manufacturers with the tools to deliver
the right product to the right patient at the right time.
According to TrakCel,
costs can also be reduced by maximizing the available assets and optimizing
headcounts necessary in the supply chain. For autologous products, where two
procedures are necessary, it will be possible to collect the sample from the patient
in a hospital nearby and limit the travel to a specialized hospital for the
treatment with the finished product. More flexibility will also come from a
longer shelf life. Shelf life as short as 18 hours are not commercially viable
for large patient populations. Even by using specialized express couriers, the
risk of failure linked to scheduling can be very high. More innovative ways to
increase the length of the shelf life are under development. Research groups
are even going as far as lyophilize the product.
So far it has been mainly
applied to red blood cells preservation. If this will ever be possible with
other cell types, it can be a game changer in the industry. Nurses and doctors
could easily re-suspend the product as they are used to doing with more
conventional drugs, but perhaps this is a topic for another blog post.
Fabio D’Agostino is a passionate life sciences professional with experience in both the medical device and biopharmaceutical industry. An active member of the PDA Cell and Gene Task Force, he has contributed to a number of conferences in the cell and gene therapy industries. He was also instrumental in the launch of the new journal: Cell and Gene Therapy Insights.
After graduating with Honours from the Polytechnic University of Turin (Italy) with a BSc and a Master’s in Biomedical Engineering, he started his career at LivaNova (formerly Sorin Group) before moving to Newcastle University to take an Engineering Doctorate in Biopharmaceutical Process Development. He currently holds a research position at the Institute of Genetic Medicine (Newcastle University) where he is responsible for the development of an innovative platform for modular tissue engineering.
|
Share this article with your social network, just click below to share now!
|
|
|






No comments :
Post a Comment